Harris D. Bernstein, Ph.D.

Professional Experience
- Postdoctoral fellow, University of California San Francisco, 1987-1992
- Ph.D., Massachusetts Institute of Technology, 1987
- B.A., Harvard University, 1980
Research Goal
My laboratory has a long-standing interest in understanding how proteins are inserted into or transported across the cell membranes of both pathogenic and non-pathogenic bacteria.
Current Research
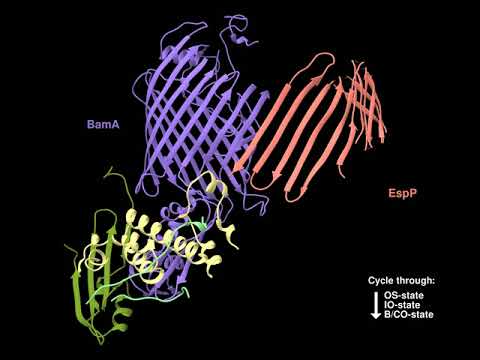
In one project we are investigating the assembly (folding and membrane insertion) of proteins that reside in the outer membrane of Gram-negative bacteria. While most integral membrane proteins contain one or more α-helical membrane spanning segments, bacterial outer membrane proteins are anchored to the membrane by a single amphipathic β sheet that folds into a distinctive closed cylindrical structure called a “β barrel”. Previous studies have shown that a highly conserved heterooligomer (the barrel assembly machine, or BAM) catalyzes the integration of β barrels into the outer membrane, but the mechanism by which it promotes insertion is poorly understood. There is considerable evidence that the BamA subunit, an outer membrane protein that contains both a β barrel and several periplasmic domains that tether the other subunits, plays a central role in the insertion reaction. To gain further insight into outer membrane protein biogenesis, we recently developed a method to arrest the assembly of a native β barrel before it dissociates from BAM and solved the structure of the purified BAM-substrate supercomplex by cryo-EM. The results show that the BamA β barrel binds to the C-terminus of the substrate and then promotes its progressive conversion from an open β sheet to a barrel-like structure in a remarkable process that is influenced by membrane tension. Although it is likely that BAM catalyzes the assembly of most outer membrane proteins by the same general mechanism, we are especially interested in understanding the nuances of the assembly of a unique class of outer membrane proteins called “autotransporters”. Like other outer membrane proteins autotransporters contain a transmembrane β barrel domain, but they also contain a large extracellular domain that often encodes a virulence function.
Because BamA is a surface localized protein that is essential for viability in E. coli and other Gram-negative bacteria, it is an extremely attractive target for novel antibiotics. Indeed several natural products and synthetic compounds have recently been reported that inhibit BamA activity and exhibit strong bactericidal activity. In a second project we are characterizing the mode of action of previously described BamA inhibitors, and through collaborative efforts we are isolating and studying new types of BamA inhibitors that have unique properties.
Finally, we are investigating proteins that belong to the same superfamily as BamA (the “Omp85" superfamily), many of which are poorly characterized or have no known function. We are particularly interested in elucidating the function of TamA, a protein that is strikingly similar to BamA but that forms a heterodimer with an elongated periplasmic protein called TamB that bears no resemblance to the other BAM subunits. The TamA/TamB complex (also known as the translocation and assembly module, or TAM) is less widely distributed than BAM, but is essential for virulence or colonization in some Gram-negative bacteria. Although genetic studies have implicated TAM in both outer membrane protein assembly and phospholipid transport from the inner membrane to the outer membrane, we recently obtained the first direct evidence that TAM functions as an outer membrane protein insertase. In a separate study, we recently found that a protein produced by the opportunistic pathogen Pseudomonas aeruginosa called PlpD, which is the prototypical member of another Omp85 family, has a different topology, oligomeric state, and function than previously proposed.
Applying our Research
Because previous research has provided a “proof of concept” that BamA is a promising target for the development of a novel class of antibiotics, our work has the potential to lead to the discovery of optimized BamA inhibitors that will help to combat the rise of multidrug-resistant Gram-negative “superbugs”. Furthermore, our work on autotransporters may accelerate the development of vaccines against specific bacterial pathogens. Autotransporters are excellent vaccine candidates because they contain a large extracellular domain, and one autotransporter (pertactin) is already in use in pertussis vaccines. Our studies on autotransporters may also lead to improvements in “autodisplay” technology, a method in which autotransporters are used for the cell surface presentation of heterologous peptides or proteins. Autodisplay has proven to be a valuable alternative to phage-display technology and may ultimately be useful as a method to present antigens to induce immune protection. Finally, our work on some of the more enigmatic members of the Omp85 superfamily of proteins might lead to surprising new insights into prokaryotic biology as well as new strategies to treat or prevent diseases caused by specific pathogens.
Select Publications
- The translocation assembly module (TAM) catalyzes the assembly of bacterial outer membrane proteins in vitro.
- Wang X, Nyenhuis SB, Bernstein HD.
- Nat Commun (2024 Aug 23) 15:7246. Abstract/Full Text
- Cryo-EM structures reveal multiple stages of bacterial outer membrane protein folding.
- Doyle MT, Jimah JR, Dowdy T, Ohlemacher SI, Larion M, Hinshaw JE, Bernstein HD.
- Cell (2022 Mar 31) 185:1143-1156.e13. Abstract/Full Text
Research in Plain Language
Living cells can be thought of as extremely sophisticated machines that only function if all of the parts are in the right place. Imagine a car in which the steering wheel is in the trunk and the wheels are on the roof. Such a vehicle wouldn’t be very useful!
Most biochemical reactions are performed by proteins, which are manufactured inside living cells. In addition to folding into a defined three-dimensional shape, many proteins must be transported across one or more membrane barriers or embedded in a membrane in order to reach the location where they perform their jobs. We are currently studying protein localization in Gram-negative bacteria, a large group of unicellular organisms (some of which cause disease) that are bounded by a double membrane. We are especially interested in understanding the process by which proteins that must be transported across the first or “inner” membrane and then inserted into the second or “outer” membrane are localized. It is now clear that a multi-protein complex called the “barrel assembly machine” or BAM that is produced by all Gram-negative bacteria drives the insertion of proteins into the outer membrane. Bacteria cannot survive if BAM is inactivated because it mediates a critical cellular process, and several natural products and synthetic chemical compounds have recently been identified that kill bacteria by inactivating BamA, the most important subunit of BAM. We hope that both by better understanding the function of BAM and by identifying new types of compounds in chemical screens that inactivate BamA we will be able to develop a new class of potent antibiotics that are optimized to treat bacterial diseases in humans and animals. Furthermore, in some of our experiments we study a specific group of outer membrane proteins called “autotransporters” that are produced by disease-causing bacteria and that are of particular interest because they can be used to generate vaccines. Autotransporters can also be modified to display other proteins on the surface of bacteria and consequently have many potential applications in diagnostics and biotechnology (e.g., bioremediation). In addition, we are studying a group of bacterial proteins that are structurally related to BamA but whose function is poorly understood or that have no known function. We hope that by elucidating the role that these proteins play in cell physiology we will be able to devise new strategies to treat bacterial infections.
Research Images