John Louis Medabalimi, Ph.D. (alias: John M. Louis)
Professional Experience
- Head, Protein Engineering and Chemistry Group, Laboratory of Chemical Physics, NIDDK, NIH, 2005-present
- Research Biologist, Laboratory of Chemical Physics, NIDDK, NIH, 1996-2005
- Staff Fellow, Laboratory of Cellular and Developmental Biology, NIDDK, NIH, 1990-1996
- Fellow, Division of Cancer Biology and Diagnosis, NCI, NIH, 1986-1989
Research Goal
A major goal of our research is to describe the intricate basic mechanisms of intracellular and extracellular macromolecular interactions in human cells and associated organisms. Understanding how normal functions are perturbed in disease conditions leads to the development of new therapies, thus, benefiting health and longevity.
Current Research
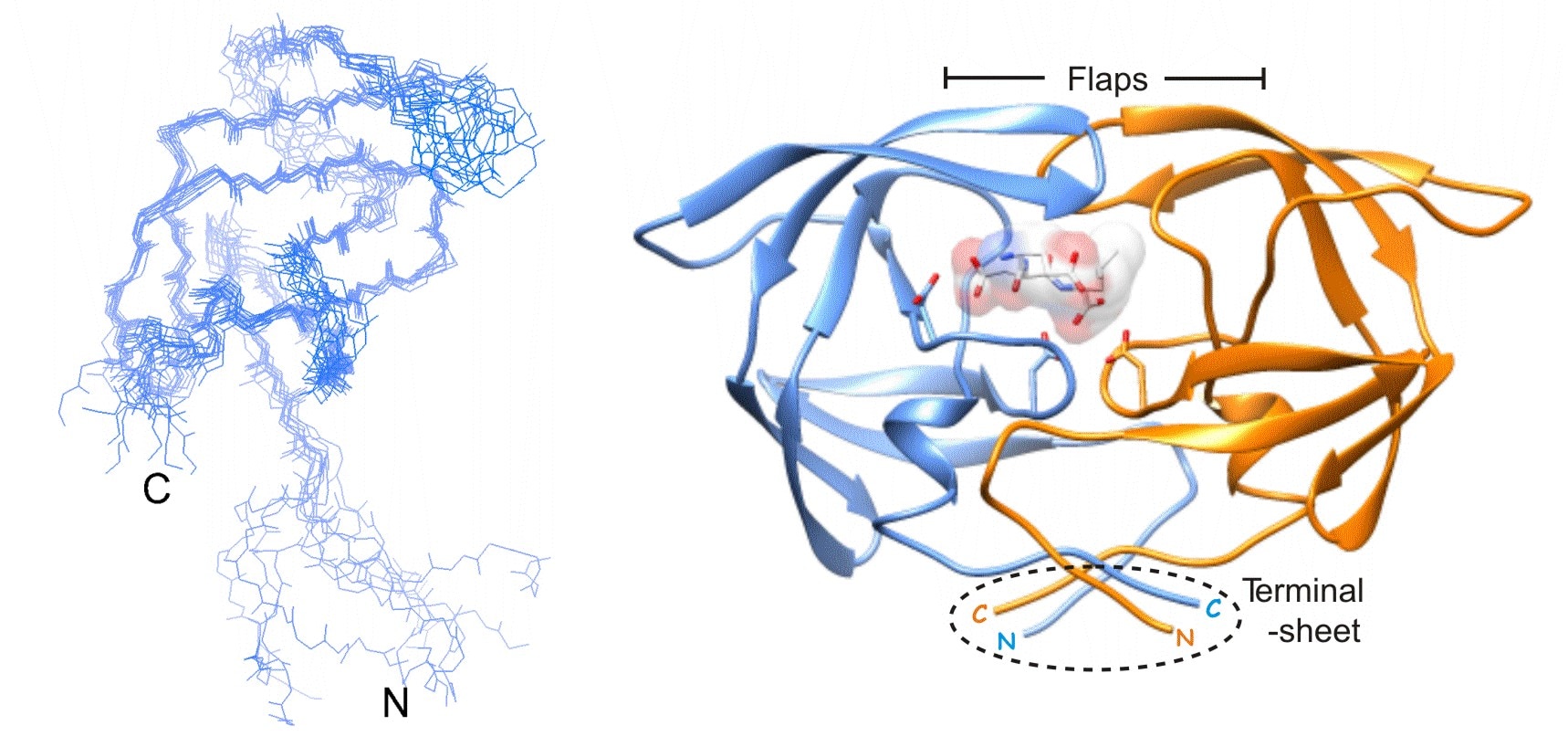
One of our primary goals is to develop and apply a variety of genetic- and protein-based technologies to optimize proteins, protein-protein/protein-nucleic acid complexes and membrane protein/detergent complexes to enable their biophysical characterization and structure determination by solution nuclear magnetic resonance (NMR). Examples include complexes involved in signal transduction and transcriptional regulation, and viral proteins. Other applications for characterization include enzyme kinetics, calorimetry, advanced optical spectroscopy and immunochemistry to elucidate reaction mechanisms and protein-protein and protein-small molecule interactions.
By interacting closely with other groups within the Laboratory of Chemical Physics, we also develop approaches for surface immobilization, protein ligation, and site-specific multiple labeling of proteins and nucleic acids with probes to enable pioneering studies of folding and dynamics by single-molecule FRET spectroscopy and NMR. Additionally, we pursue long-term basic studies on specialized aspects of regulation of retroviral proteases, with the goal of developing new concepts for therapy.
Applying our Research
Advances in retroviral therapy have enabled the long-term survival of HIV patients. However, error-prone replication of the viral genome results in high mutational frequency and the rapid selection of resistant mutants under drug pressure. We are investigating approaches that target initial events in retroviral protease maturation. The outcome of these studies may provide novel alternative strategies to circumvent drug resistance.
Need for Further Study
Additional studies are needed on thermodynamic characterization of antigen-antibody reactions in conjunction with mutagenesis of engineered single-chain antibodies to understand the molecular basis for viral neutralization. This knowledge may contribute to development of effective vaccines and/or immunotherapy for HIV and other viral infections.
Select Publications
- Three-Color Single-Molecule FRET and Fluorescence Lifetime Analysis of Fast Protein Folding.
- Yoo J, Louis JM, Gopich IV, Chung HS.
- J Phys Chem B (2018 Dec 13) 122:11702-11720. Abstract/Full Text
- Co-Evolutionary Fitness Landscapes for Sequence Design.
- Tian P, Louis JM, Baber JL, Aniana A, Best RB.
- Angew Chem Int Ed Engl (2018 May 14) 57:5674-5678. Abstract/Full Text
Research in Plain Language
We design, optimize, and develop methods to change proteins and nucleic acids to enable studies of the physical and structural properties that are essential for their function. We study the function of biological molecules, which participate in communication within and between cells, in gene expression and in regulating the replication and spread of viruses.
Research Images