About Our Research
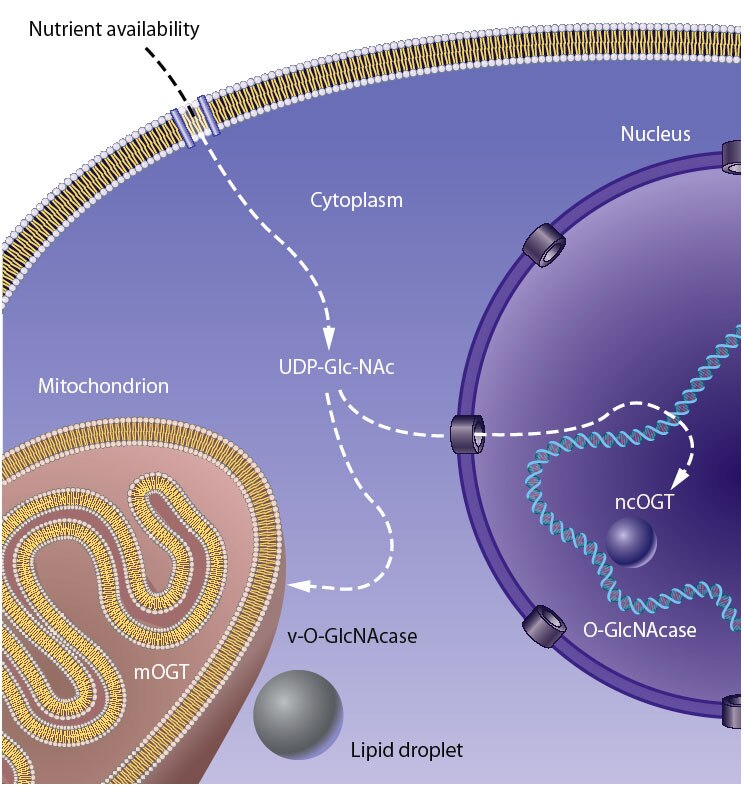
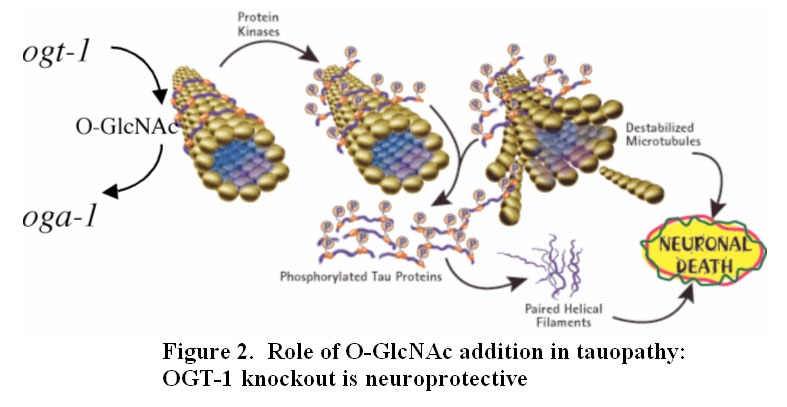
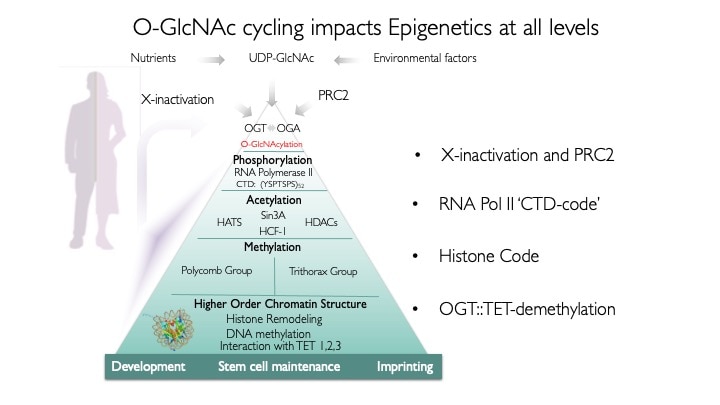
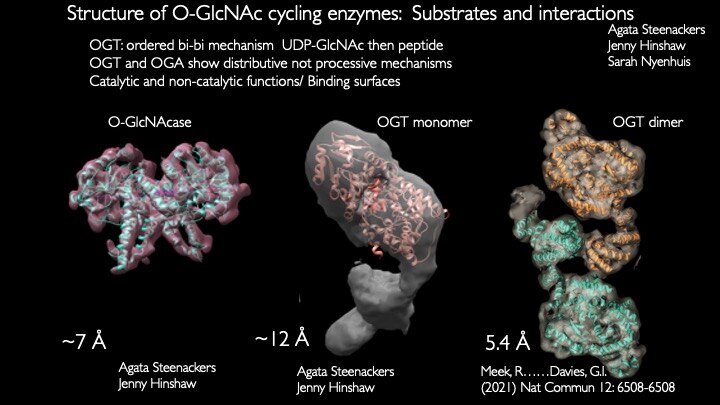
Our laboratory seeks to understand how nutrient-responsive O-GlcNAc cycling influences cellular signaling, gene expression, and epigenetics.Our long-term goal is to define the role of O-GlcNAcylation in chronic human disease.Integrating cellular glucose, amino acids, and lipids, the hexosamine-signaling pathway (HSP) terminates in O-GlcNAc cycling, a key sensor of cellular nutrient availability.O-GlcNAc transferase (OGT) utilizes the terminal product of the HSP, UDP-GlcNAc, to modify many key targets including enzymes, signaling kinases, nucleoporins, and transcription factor. O-GlcNAcase (OGA) catalyzes O-GlcNAc removal. Unlike canonic endomembrane glycosylation, this dynamic cycle of O-GlcNAc addition and removal occurs in the nucleus, cytoplasm, and mitochondria due to differentially targeted variants of OGT and OGA.
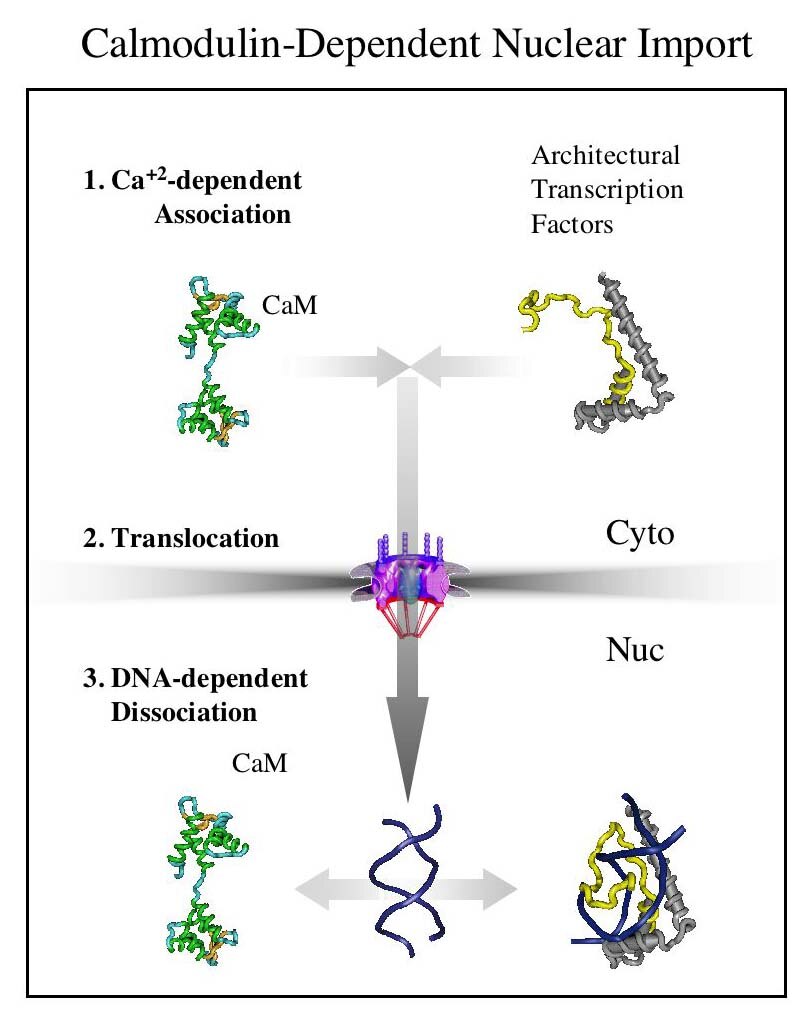
Our hypothesis is that differentially targeted isoforms of the enzymes of O-GlcNAc metabolism mediate a glycan-dependent signaling pathway broadly impacting signaling and the epigenetic regulation of gene expression. We argue that the enzymes of O-GlcNAc cycling function as a nutrient-responsive homeostatic module critical for key metabolic and cell fate decisions. Responding to nutrient levels and other environmental cues, O-GlcNAc cycling impacts growth factor signaling, differentiation, stem cell maintenance and epigenetic regulation of gene expression.
The role of O-GlcNAcase in maintaining metabolic homeostasis
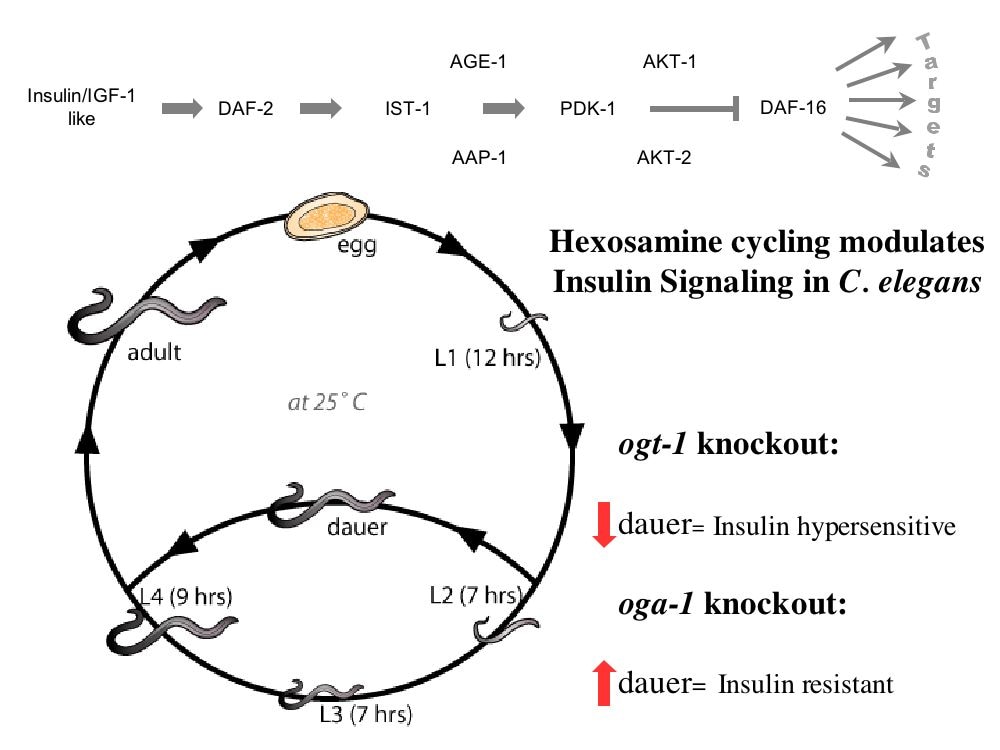
In C. elegans we found that O-GlcNAc cycling acts with other nutrient sensors including NHR-49 (PPAR homolog) to trigger ARD entry and maintenance. O-GlcNAc cycling acts upstream of this worm PPAR homolog to regulate ARD through fat storage and lipid b-oxidation. We have generated an O-GlcNAcase knockout (OgaKO) mouse model to extend our genetic observations in the worm and fly models. Upon germline deletion, knockout mice have a perinatal lethality phenotype with only about 3% surviving into adulthood, low circulating glucose and low liver glycogen stores. Transcriptomics analysis suggested a widespread deregulation of growth and metabolic genes. Both surviving knockout and heterozygous mice had increased fat metabolism, reduced insulin sensitivity, glucose tolerance and hyperleptinemia. In a transcriptomics analysis of livers and hearts of neonatal mice, we found that PPAR a and g-dependent transcription is massively deregulated in the OgaKO mice leading to altered mitochondrial b-oxidation, fat storage and suppression of transcription of the endocrine growth factor FGF-21. We are now examining the epigenetic and signaling cascades responsible for the OGA-driven metabolic dysregulation with a focus on the regulation of FGF-21 expression.
Development, stem cell Biology, epigenetics and immunity
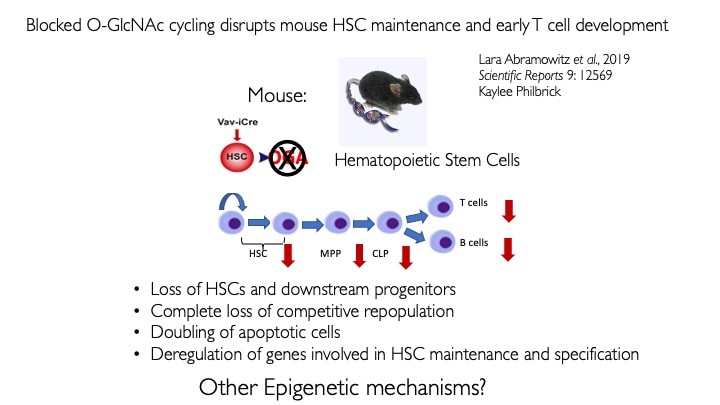
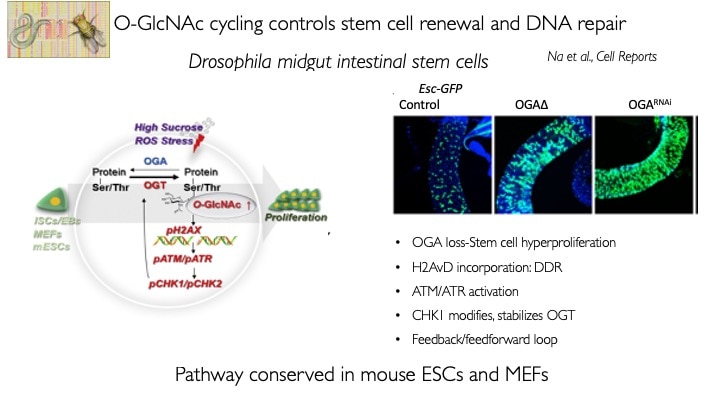
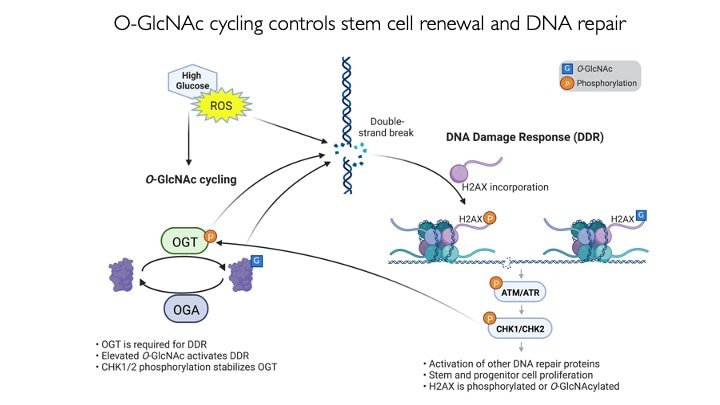
We use animal models with altered O-GlcNAc cycling for developmental and genomic studies. The C. elegans and Drosophila models allowed dissection of the role of O-GlcNAc in male fertility, polycomb repression and trithorax activation. In the Drosophila intestinal stem cell system, we showed that loss of OGA is associated with enhanced proliferation and acts upstream of activation of ATM/ATR-dependent double strand breakpoint DNA repair (DSB). This activation of DNA damage repair is conserved in MEFs lacking O-GlcNAcase. Thus, the enzymes of O-GlcNAc cycling may be key regulators of the DNA damage repair pathway. These finding have obvious implications for the role of O-GlcNAc in cancer and stem cell biology. In the mouse, we have explored each of the phenotypes observed in germline Oga mutants using more targeted conditional Cre-knockouts. Knockout of Oga at early and late stages of hematopoietic development results in loss of long-term hematopoietic stem cells and early lymphoid precursors. Further, we found that when stimulated OGA ko macrophages produce increased levels of proinflammatory cytokines like Il6. An understanding of how nutrient-driven O-GlcNAc cycling maintains stem cell self-renewal and pluripotency is critical for future applications in pathophysiology, immunology and stem cell-based therapies.
Tool development for specific detection of glycans
Our lab has been developing bioorthogonal sugars for specific detection of diverse classes of glycoconjugates. We have identified probes specific for O-GlcNAc glycoproteins, N-glycan modified proteins and glycolipids. We are exploring the use of Raman microscopy coupled with fluorescence microscopy to examine the biogenesis, localization and function of the various glycan species.
Research Images