About Our Research
Chronic viral hepatitis ranks as the seventh leading cause of death globally. Worldwide, there are an estimated 292 million persons with chronic hepatitis B (CHB) and 71 million persons with chronic hepatitis C (CHC). Both infections account for over 1.3 million deaths annually due to complications from liver cirrhosis and liver cancer. Notably, the prevalence of CHB remains high despite the availability of an effective vaccine. These grim statistics serve to underscore the magnitude and impact that chronic viral hepatitis has on global and U.S. public health. Consequently, in 2016 the World Health Organization issued a recommendation to eliminate hepatitis B and C by 2030 that was endorsed by the National Academies of Sciences, Engineering and Medicine.
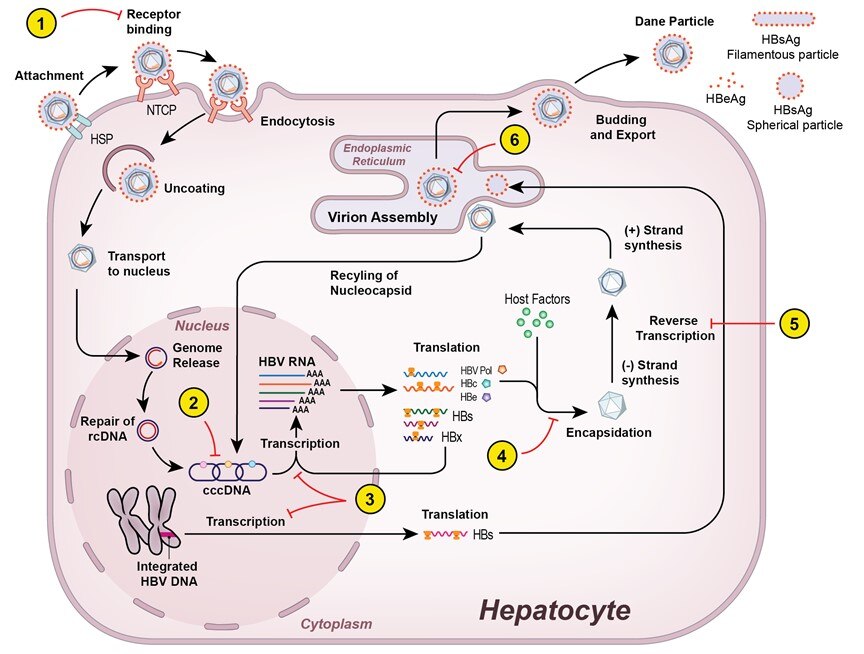
Significant knowledge gaps in natural history and treatment of CHB exist. In particular, not all patients need to be treated and identifying those who benefit the most from treatment are important areas of research. Current therapy for CHB suppresses viremia but is not curative, therefore, it must be administered long-term. Given the high burden of CHB on public health, there is an urgent need to develop safe, finite duration, curative therapies. In contrast, treatment of CHC has been revolutionized by the development of safe and highly effective direct acting antiviral agents (DAAs). However, complications may still occur after curative therapy. Thus, prospective studies to identify factors that influence long term outcome particularly related to development of liver cancer after curative therapy are warranted. My research program is centered around these important clinical issues.
Specific goals:
- Define the host and viral factors that determine the outcome of CHB and cured CHC.
- Develop and evaluate novel, safer and more effective regimens for CHB.
- Elucidate mechanisms of action of therapy and predictors of treatment response for CHB.
Approaches
Our research team conducts long-term outcome and proof-of concept studies that provide opportunity for translational research, for which the existing NIH infrastructure is ideally conducive. Within this environment, the team has collaborated extensively with basic scientists in the Liver Diseases Branch (LDB) and NIH to complement our clinical research studies. These pilot studies are meant to spawn larger randomized efficacy studies or to address important deficiencies of ongoing pharmaceutical trials. Additionally, the team is involved in large national, multicenter studies such as the Hepatitis B Research Network (HBRN) where I serve as the Primary Investigator for the LDB site. Our participation in these studies bridges the NIDDK Intramural Research Program with academia and allows the opportunity to collaborate with extramural investigators. These multiple approaches are synergistic, whereby feasibility studies conducted at the NIH may be extended to larger phase 2-3 extramural multicenter studies and bench-to-bedside collaborations inform new pilot studies. The research team has taken the following approaches:
- Longitudinal retrospective-prospective studies to identify factors that affect the outcome of CHB and cured CHC and to develop prediction models to assess disease severity and outcome.
- Design and conduct clinical trials that incorporate innovative approaches to prevent or limit disease progression in CHB.
- Conduct translational studies using biospecimens collected from the clinical trials to better understand disease outcome, treatment response and to provide insights into future, novel therapeutic approaches.
Research Images