Virtual Subgroup Meeting - January 19, 2022
Urine Albumin Standardization Status Update Meeting
The meeting is a joint activity with the International Federation of Clinical Chemistry (IFCC) Working Group on Standardization of Albumin in Urine. This is a meeting of a sub-group directly involved with the reference measurement procedures and reference materials for standardization of urine albumin to plan completion of JCTLM submission readiness.
Meeting participants: Lorin Bachmann, Ashley Beasley-Green, Qinde Liu, Greg Miller, Jenna Norton, Afshin Parsa, Karen Phinney, Jesse Seegmiller, John Lieske
Welcome and Meeting Purpose
Greg Miller, Virginia Commonwealth University
This small subgroup meeting is in lieu of the larger laboratory working group meeting and includes only those actively participating in urine albumin standardization efforts. The focus of the meeting will be on completing the standardization of urine albumin. The primary objectives for the meeting are to 1) understand the status of efforts to submit urine albumin reference materials and procedures to the Joint Committee for Traceability in Laboratory Medicine (JCTLM) and 2) determine the next steps for designing and completing the urine albumin round-robin study. A written summary will be provided to the full Laboratory Working Group, and he requested that all labs participating in the urine albumin standardization efforts provide a written status update to assist with the development of that summary.
Certified reference materials (CRM) for urine albumin status update
Karen Phinney and Ashley Beasley-Green, NIST
NIST SRM 3666 Albumin in Frozen Human Urine. NIST is well underway with NIST 3666. Preliminary assessments are complete and values for albumin in urine are consistent with target values. NIST is moving forward with certification and stability testing using the NIST reference measures procedure (RMP), and then will proceed with the commutability assessment. The final value assignment was delayed due to COVID, but will be completed in the very near future.
Commutability assessment plan for SRM 3666. The group discussed plans for the commutability study, which will include an initial pilot study involving 5 or 6 laboratories with a limited number (~10) of clinical samples. If the pilot study is successful, it will be scaled up into a full commutability study. NIST has a quality assurance program (QAP) platform for clinical analytes that can be used for both pilot and full studies to allow participants to upload data and acquire data from participants as well as to manage infrastructure (e.g., shipping labels). Pilot samples will be assessed at the following labs: Mayo (using Roche), Minnesota (using Siemens), and Virginia Commonwealth University (VCU) (using Abbott). Labs using Beckmann and Ortho analyzers will need to be recruited. It may be practical to do the full commutability study in combination with round robin study for reference method comparison. Additional next steps include preparing a study protocol, determining shipping logistics, and identifying a lab to source the urine samples – potentially VCU or Minnesota. Dr. Miller will lead development of the protocol with input from with Drs. Seegmiller, Bachmann, and Liu.
The Singapore Health Sciences Authority (HSA) obtained data for a commutability study in December 2021 by collaborating with 4 clinical laboratories from local hospitals. One hospital provided ~25 clinical samples and the other 3 measured both patients samples and the CRM across major clinical analyzer brands including Beckman, Abbott, Roche, and Siemens. The Singapore HSA is in the process of analyzing data. The Laboratory Working Group will follow a similar procedure in the US, and the Singapore HSA may be able to provide insights.
Preliminary Comparison between NIST and NMIJ Albumin Solution CRMs
Qinde Liu, Singapore Health Sciences Authority
Both the U.S. National Institute of Standards and Technology (NIST) and the National Metrology Institute of Japan (NMIJ) have developed human serum albumin solution certified reference materials (CRM) as primary calibration standards. NIST SRM 2925 is recombinant human serum albumin, while NMIJ CRM 6202-a is human serum albumin from pooled serum. There may be some difference between these two primary albumin standards. HSA has used NMIJ CRM 6202-a as the calibration standard to certify albumin in urine CRMs and to assign the values of albumin in urine for external quality assessment (EQA) samples. As NIST SRM 2925 is intended for use as the calibration standard for urine albumin standardization, preliminary comparison between NIST SRM 2925 and NMIJ CRM 6202-a was conducted via both amino acid analysis and trypsin digestion method.
When comparing molecular mass of NIST SRM 2925 and NMIJ CRM 6202-a using high resolution mass spectrometry, results suggest NMIJ CRM 6202-a has a slightly larger molecular mass than NIST SRM 2925. The center of mass distribution for NIST SRM 2925 is close to the relative mass in the certificate of analysis; however, the center of distribution for NMIJ CRM 6202-a is larger than the relative mass in the NMIJ certificate of analysis because NMIJ calculated the relative mass based on albumin sequence in the certificate of analysis. The difference is likely due to glycosylation of urine albumin. Of the eight peptides used for quantification after trypsin digestion, six may be affected with possible glycosylation based on residuals reported in literature.
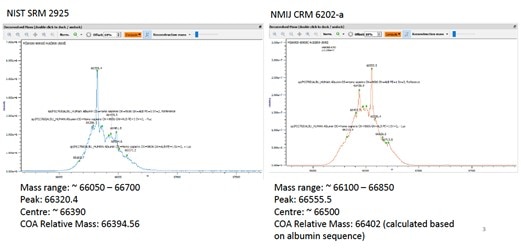
The certified concentrations of both NIST SRM 2925 and NMIJ CRM 6202‐a were confirmed using isotope dilution mass spectrometry amino acid analysis. Five amino acids (valine, proline, leucine, isoleucine, and phenylalanine) were used for quantification. The following amino acid CRMs from the Singapore Health Sciences Authority were used as the calibration standards:
HRM‐1006A L‐valine, HRM‐1007A L‐proline, HRM‐1008A L‐leucine, HRM‐1013A L‐isoleucine, and HRM‐1014A L phenylalanine. All of these amino acid CRMs are primary calibrators listed in the Joint Committee for Traceability in Laboratory Medicine (JCTLM) database. The values obtained for both NIST SRM 2925 and NMIJ CRM 6202‐a are well within the certified uncertainty ranges of the respective CRMs, suggesting that certified concentrations of both NIST SRM 2925 and NMIJ CRM 6202‐a are accurate.
| NIST CRM 2925 | NMIJ CRM 6202-a | |
|---|---|---|
| Certified value and uncertainty (nmol/g) | 14.4 ± 0.3 | 1097 ± 31 |
| Relative uncertainty of certified value | 2.1% | 2.8% |
| Obtained value (nmol/g) | 14.14 | 1113 |
| Relative deviation from certified value | -1.83% | -1.34% |
Trypsin digestion was used to cross verify the two CRMs. When NIST SRM 2925 was used as a calibration standard to measure NMIJ CRM 6202‐a, the relative deviation was -7.5%. When NMIJ CRM 6202‐a was used as a calibration standard to measure NIST SRM 2925, the relative deviation was +9.7%. This suggests a slight difference between the two CRMs, with obtained values for NIST SRM 2925 slightly higher than certified values and obtained values for NMIJ CRM 6202‐a slightly lower than certified values.
HRM-3004A was created as a CRM for urine albumin with certified values determined using NMIJ CRM 6202‐a as a calibration standard. NIST SRM 2925 was used as a calibration standard to measure HRM-3004A to see if certified values could be reproduced. Results varied substantially across the two calibration standards, and the reasons for these differences are not yet understood. Of note, this analysis was conducted on a small number of samples and thus a more robust analysis may be necessary. However, obtained values are well within uncertainty ranges of the certified values. Once an EQA sample is assigned using a urine CRM as the quality control, which is expected to occur in April/May 2022, the 2 CRMs can then be compared using two sets of calibration standards.
This work indicates that the certified values of both NIST SRM 2925 and NMIJ CRM 6202‐a are accurate based on IDMS amino acid analysis. While some uncertainty remains, cross verification between NIST SRM 2925 and NMIJ CRM 6202‐a using trypsin digestion showed differences between these two CRMs. It is likely attributed to the different sources of the materials (recombinant vs pooled human serum). Using NIST SRM 2925 as calibration standard, the certified values of the urine CRM (HRM‐3004A) can be reproduced, although the certified values were determined using NMIJ CRM 6202‐a as calibration standard.
Discussion
Dr. Miller suggested that findings indicate that both NMIJ and NIST materials are acceptable calibrators.
Dr. Phinney noted that it is not surprising to see some differences using a peptide method, as it is hard to account for variations in protein completely. Thus, the differences are not troubling.
Dr. Miller raised the question as to whether this agreement would be considered acceptable in JCTLM review. Dr. Phinney noted that the question of equivalence has not been fully addressed. NIST SRM 2925 has been submitted to JCTLM, but comments have not yet been received from the review team.
Reference Measurement Procedures (RMP) for Urine Albumin
Status toward publication of performance and readiness for JCTLM submission
NIST. Method validation for the NIST RMP is complete and published, and they have moved on to certification.
Beasley-Green A., Burris N., Bunk D.M., Phinney K.W. “Multiplexed LC-MS/MS Assay for Urine Albumin”. J. Proteome Res. 2014, 13, 9, 3930-3939.
Minnesota. The candidate RMP was validated to better understand imprecision in single measurements for all six included albumin peptides independently. The validation found the candidate RMP performed at 4-8% CV throughout the calibration range of 3-200 mg/L. However, the NIDDK Laboratory Working Group previously determined the RMP would need to perform at < 5% CV. Prior consensus was to use replication of N=4 to achieve the targeted level of precision of < 5%. NIST SRM 2925 was obtained, but at low volumes making it infeasible to produce calibrators lasting one to two years from that material. Instead, in-house calibrators were prepared from Sigma Aldrich human serum albumin, which is stated at 99% pure. Target concentrations were made from this sample ranging from 3 to 200 mg/L. When NIST SRM 2925 and in-house calibrators were compared, results were discrepant by as much as 25%. Sigma Aldrich human serum albumin starting material may contain different levels of fragmented human serum albumin that may be causing this discrepancy. Therefore, Minnesota has identified an alternative source of albumin from Albumin Biosciences. Albumin Biosciences-derived calibrators yielded results closer to expected values. In conclusion, the LC-MS/MS candidate RMP validation with quadruplicate replication is complete. Value assignment of laboratory calibrators will occur using the NIST SRM 2925. Sample comparisons among the candidate RMPs will follow as laboratories come online, and Minnesota is ready for the round robin study. Because independent publication of each RMP is optimal for JCTLM submission and enables future users to easily reference the RMP, Minnesota will work toward publication of this data prior to a May 2023 JCTLM submission.
Mayo. No work has occurred in this in the last year due to COVID. Mayo plans to reproduce the Minnesota methods, with the only differences being equipment and location. Given the similarity in methods, Minnesota and Mayo may publish jointly.
Health Sciences Authority. The Singapore HSA RMP was published in Clinical Chemistry and Laboratory Medicine. The initial RMP used NMIJ CRM 6202‐a as a calibration standard, since NIST SRM 2925 was not yet developed. Next steps will involve comparing NIST SRM 2925 and NMIJ CRM 6202‐a as the calibration standard. Dr. Seegmiller expressed interest in getting HRM-3004A material from Dr. Liu in order to compare in his analyses.
Round Robin to Compare Results from RMPs
Dr. Miller noted that a round robin study to compare differences across the four RMPs would be needed for JCTLM submission. He proposed an initial pilot study with a small number of samples (~10-15) to identify any potential significant disagreement between the RMPs. The group discussed details of the round robin, including timing, use of fresh or frozen samples, number of samples, and aliquot volume. The Round Robin could be launched within approximately one month (late February/early March 2022). Drs. Phinney and Beasley-Green noted that NIST may need an additional month in order to schedule time on requisite instruments. NIST will follow up with a reasonable time frame for their participation. Lead time may be required for all labs to allow for acquiring supplies, as major suppliers have backlogs for necessary materials, as well as to allow time for shipping. The group agreed to frozen samples due to challenges shipping fresh samples and the greater flexibility in timing with frozen samples—allowing each lab to schedule at their earliest convenience.
Discussion suggested that 10 samples covering the measuring intervals of the RMPs would be sufficient for JCTLM submission. This was confirmed by Drs. Liu and Phinney, who have substantial experience reviewing for JCTLM. Rather than emphasizing the number of samples, JCTLM will give more scrutiny to potential impacts on the clinical community. To confirm sample number is sufficient, Dr. Miller will contact Jeff Budd to conduct power calculations for number of samples and replication to identify 5% bias at 30 mg/L. Before sending, he will draft a request and send to the group for input. If issues are identified in the initial round of 10 samples, the study may need to be repeated after corrective action is taken.
The necessary aliquot volume of the samples will depend on number of replicates needed for the study, and will vary from lab to lab. The approximate concentration from clinical lab measurement procedures can be provided, and thus this analysis will not need to be performed at each lab. As a result, the aliquot volume does not need to contain sufficient volume for each lab to conduct their own rough values. Therefore, samples from 2-3mL would be sufficient. Whichever lab completes the clinical analysis will receive an additional vial. Dr. Millers lab could perform a clinical analysis using Abbott and Dr. Liu’s lab could perform a clinical analysis using Roche. The group briefly debated whether it would be efficient to combine the commutability and round robin studies, but concluded that sample volumes would likely not be sufficient to combine the studies.
Drs. Miller and Bachman will develop the round robin protocol and circulate to the group for input. Once the protocol is developed, Dr. Bachman will work with Dr. Miller to identify estimated costs and will seek funding to support costs including shipping from the International Consortium for Harmonization of Clinical Laboratory Results (ICHCLR). Costs will include aliquot containers, labels, shipping containers, dry ice, and shipping within the US and to Singapore.
This content is provided as a service of the National Institute of Diabetes and Digestive and Kidney Diseases
(NIDDK), part of the National Institutes of Health. NIDDK translates and disseminates research findings to increase knowledge and understanding about health and disease among patients, health professionals, and the public. Content produced by NIDDK is carefully reviewed by NIDDK scientists and other experts.

