News Around NIDDK
NIDDK director testifies on type 1 diabetes research
By Dr. Mary Hanlon

On July 10, NIDDK Director Dr. Griffin P. Rodgers testified about progress in type 1 diabetes research before the Senate Special Committee on Aging, which is led by Chairman Bill Nelson (D-FL) and Ranking Member Susan Collins (R-ME). The hearing, entitled “Diabetes Research: Reducing the Burden of Diabetes at All Ages and Stages,” was held in conjunction with the Children’s Congress, an event sponsored by the JDRF (formerly called the Juvenile Diabetes Research Foundation).
Testifying with Rodgers were National Basketball Association star Ray Allen and his 6-year-old son, Walker, who was diagnosed with type 1 diabetes at 17 months of age; Emmy Award-winning actress Jean Smart, who was diagnosed with the disease at age 13; JDRF President and CEO Jeffrey Brewer, also the father of a child with type 1 diabetes; and 14-year-old Quinn Ferguson, a JDRF Children’s Congress delegate and participant in an NIDDK-supported clinical trial being conducted by Type 1 Diabetes TrialNet investigators.
In his testimony, Rodgers described recent NIH- and NIDDK-supported advances and future opportunities in type 1 diabetes research, including research supported by the Special Statutory Funding Program for Type 1 Diabetes Research (Type 1 Diabetes Special Statutory Funding Program).
“The future for those with type 1 diabetes is brighter than ever,” Rodgers said, giving examples including the recent finding that intensive glucose control early after type 1 diabetes diagnosis can prevent the development and slow the progression of diabetic kidney disease by 50 percent. He said NIDDK is building on that and other recent progress and remains steadfast in its dedication to support research toward identifying ways to prevent, treat and ultimately cure type 1 diabetes.
“Together, we strive to allow those of all ages with type 1 diabetes to live long, healthy lives free of the burden of disease.”
Read Rodgers’ testimony. A webcast of the hearing is available on the website of the Senate Special Committee on Aging.
Three decades later, diabetes study volunteers still find reward in participation
By Amy F. Reiter

Thirty years ago, Elizabeth Rude joined a type 1 diabetes study called the Diabetes Control and Complications Trial so she could get the free supplies. What she received from participating in the NIDDK-funded DCCT and its follow-up, Epidemiology of Diabetes Interventions and Complications, was so much more.
“I am a much healthier person because of participating in this study,” Rude said. “I have had type 1 diabetes for 38 years, and I have no complications. I attribute this to being on intensive insulin therapy for so long and having such excellent support from the folks at the study.”
Her fellow participant Judi Boland concurs. “I have learned so much about my condition and how to take the best care of myself to avoid complications,” she said.
In addition to improving her own health, her participation and that of the other study volunteers has paved the way to crucial knowledge about the long-term effects of early intensive treatment for type 1 diabetes.
At a symposium for the 30th anniversary of the DCCT/EDIC, held as part of the American Diabetes Association’s Scientific Sessions in late June, Rude and many other DCCT/EDIC participants—along with hundreds of diabetes scientists and practitioners—heard the latest results of the study. Nearly two decades after the randomized trial ended and all participants were transitioned into intensive therapy, participants initially in the intensive therapy group were about 60 percent less likely to have heart disease and stroke, 50 percent less likely to have impaired kidney function, and 50 percent less likely to be in the severe, vision-threatening stages of diabetic eye disease.
“By following people for 30 years, we’ve found out incredibly important things, both about the effects of the therapy, and also about the course of type 1 diabetes in today’s world with contemporary therapy. The results are very hopeful,” said Dr. Judith Fradkin, director of the NIDDK Division of Diabetes, Endocrinology and Metabolic Diseases. “Diabetes is a lifelong disease, so what matters to people is what’s going to happen over the course of their life. With this study, we’ve shown that about 6.5 years of early intensive therapy pays continuing dividends over several decades—in healthier lives.”
DCCT participants were initially randomly divided into two groups. About half received intensive therapy, and the other half the standard therapy of the time. Rude was randomly assigned to the intensive treatment group, Boland to the standard. After the DCCT ended and EDIC began, the standard therapy group received training on intensive therapy. Both groups then received diabetes management from providers of their choosing, most using intensive diabetes treatment, which—thanks to the DCCT results—has become the improved standard treatment.
Dr. Saul Genuth, professor of medicine at Case Western Reserve University, has helped lead this study from the beginning. “This 30-year (and still counting) study shows the scientific and clinical power of the partnership between loyal research participants, investigators and sustained sponsorship by the NIDDK to improve understanding and outcomes of a once-crippling major disease,” he said.
Both Rude and Boland said the volunteer experience was rewarding in ways beyond health. Both formed a close bond with nurse Barbara Batey Schaefer at Northwestern University, one of the study sites. “Any time I have questions about my health (diabetes or otherwise), I contact her,” Boland said. “Barb came to my wedding – she is a good friend.”
Boland said she’d encourage anyone interested in volunteering for an NIH-funded clinical trial to do it. “It's worth every minute spent filling out paperwork, answering questions, getting all sorts of medical tests done,” she said. “The results of the trial might change the course of medical treatment and improve the life of someone in the future.”
For Rude, hearing the results gives her own experience even greater impact. “It is great to be reminded that what I and all the participants have done is a huge deal and has made the lives of countless others better and healthier,” she said. “How many people can say they've been a part of a study that has changed the way a disease is treated across the entire country?”
First reports from Kidney Research National Dialogue published
By Bill Polglase

If two brains are better than one for finding solutions to challenges, imagine the power of 1,600 brains.
That was the thinking behind the Kidney Research National Dialogue (KRND). Staff at NIDDK launched the interactive KRND website in 2010 as a forum for discussing directions and opportunities for kidney research. NIDDK asked scientists and others to form and prioritize research objectives to improve understanding of kidney function and disease. The KRND received input from around the nation and the world. More than 1,600 participants and 300 ideas later, the dialogue has taken shape in the form of a series of topical commentaries.
“Our goal for KRND was to develop a crowd-sourced vision for mapping and discussing the future directions of kidney research,” said Dr. Robert Star, director of the NIDDK Division of Kidney, Urologic, and Hematologic Diseases (KUH). “These commentaries were forged out of KRND’s open, non-directed online forum, and now resemble modules of a larger, planned construction project. As we started this project, we were unsure what to expect. But now we can see the beginning of an elegant skyscraper emerging, rising from a strong foundation to shape kidney research in the 21st century.”
The next step is to share the commentaries. An overview of KRND activity and commentary on diabetic nephropathy appears as a special feature in the September 2013 issue of the Clinical Journal of the American Society of Nephrology.
Topics for future commentaries, to be published separately, include: acute kidney injury, chronic kidney disease, disease education/translation, end-stage kidney disease/dialysis, glomerulonephritis/inflammation, hypertension, normal biology/development/physiology, polycystic kidney disease, training and transplantation. A final commentary will present cross-cutting themes and concluding remarks.
According to NIDDK’s Dr. Krystyna Rys-Sikora, KRND project leader, the overview commentary provides a window into the NIDDK’s first experience with web-based strategic planning. The Web approach enabled open access for ideas and conversation from a large, diverse group.
“We hope that this series of commentaries constitutes a cohesive, integrated vision of future research opportunities to be pursued by the kidney research community and supported by the NIDDK,” she said.
View more information about the KRND.
Getting to know: Dr. Allen Minton

Dr. Allen Minton is a senior investigator at NIDDK’s Laboratory of Biochemistry and Genetics. In June, he was featured in a special issue of Biophysical Reviews to honor his 70th birthday and achievements as “one of the most innovative biophysical scientists of the last 50 years.” Minton talked with Krysten Carrera about his career and accomplishments.

It’s impressive to have a well-known journal publish a special issue in your honor. How did you feel when you learned that they wanted to recognize you in that way?
Initially I was surprised and not too enthusiastic about the idea, but the editors convinced me that a lot of people have appreciated my work over the years and that this could be a fitting tribute to what I’ve accomplished alongside my very talented colleagues. It has been humbling to be honored this way.
Why did you decide to become a researcher?
I’ve always been curious about the world, specifically the lineage of scientific theories and the history of ideas. I decided to pursue science hoping to build on the ideas of others before me and perhaps come up with a few good ideas of my own. Intellectually, my greatest experiences have been those rare moments when I think I might be the only person who understands something small about the natural world. Those “eureka” moments make the sometimes tedious experiments worthwhile, and I am always eager to share what I learn with others.
What work are you best known for?
During the late 1970s and early 1980s, my coworkers and I pioneered the concept of macromolecular crowding, which describes how the presence of a high concentration of macromolecules in a particular medium influences the way each species of macromolecule behaves in that medium. I’ve also spent considerable time studying hemoglobin, sickle hemoglobin, insulin and insulin receptors. Much of my research, including what I’ve learned from interactions between pharmacologically active ligands and their cellular receptors, has set the foundation for drug developments. This is a great example of the profound, albeit indirect, effect that basic research can have on our lives.
When you first came to the NIDDK in 1970, it was known as the National Institute of Arthritis and Metabolic Diseases. Since then, what have been the biggest changes within our institute?
I was very lucky to be the beneficiary of a generous system that encouraged science for science’s sake, and I’m not sure how strongly that approach will continue in our current economic climate. But a few things about NIDDK have not changed: we still work in a relatively stable, nurturing environment filled with talented scientists. I’m extremely grateful to this institute for hanging in there with me.
What is your advice for scientists at the beginning of their careers?
Scholarship is of utmost importance because it allows you to draw upon a bounty of existing knowledge to connect the dots and form a deeper awareness of natural phenomena. Science, like art, has to come from within: you must be driven by a vision and a desire to better understand the natural world in which we live.
‘Wildly successful’ ARRA Challenge Grant researchers identify genes that cause CKD in children
By Bill Polglase
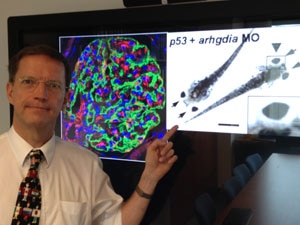
In 2009, researchers at the Howard Hughes Medical Institute received a Challenge Grant through the American Recovery and Reinvestment Act (ARRA), a 2009 program funding $8.2 billion in extramural research given to the NIH to stimulate the U.S. economy through the support and advancement of science. The Howard Hughes researchers took on a difficult task: to find genes that cause steroid-resistant nephrotic syndrome (SRNS), a frequent cause of chronic kidney disease (CKD) in children and young adults.
In summer of 2013, the researchers, led by Dr. Friedhelm Hildebrandt, announced major results. They had discovered 10 novel genes as causes of SRNS.
“No cure is available at present for SRNS, but identifying single-gene causes of the disease has provided the first fundamental insights into its disease-causing mechanisms,” said Hildebrandt. “Revealing these processes may one day lead us to therapeutic interventions that until now were considered impossible.”
SRNS is defined by protein in the urine, abnormally low levels of albumin in the blood and edema. Unlike many people with nephrotic syndrome, people with SRNS cannot rely on conventional steroid therapy, and many secondary treatments can be less effective.

Hildebrandt and his team’s three-year study analyzed DNA samples from more than 3,000 SRNS patients using techniques known as genome homozygosity mapping and whole exome resequencing, which are accelerated methods to identify, evaluate, and characterize genetic mutations. In addition, the study successfully used zebrafish to help understand the genetic mutations found in people. The scientists have already expanded and applied the new strategy of rapid gene identification to research into other hereditary conditions affecting the kidney and urinary tract.
To date, the study has generated about a dozen published articles in prominent scientific journals, including the New England Journal of Medicine. The most recent paper published, “ARHGDIA mutations cause nephrotic syndrome via defective RHO GTPase signaling,” is in the August 2013 issue of the Journal of Clinical Investigation.
“This study is just one example of the great impact ARRA funding has had on advancing research and potential treatment for serious chronic diseases,” said Dr. Rebekah Rasooly, director of the NIDDK Division of Kidney, Urologic and Hematologic Diseases Program in Genetics and Genomics. “As a result of their wildly successful efforts that met all of their key objectives, Dr. Hildebrandt and his team have identified a large number of new genes that are involved in kidney disease by operationalizing a highly efficient mutation discovery pipeline.”
The study was funded by NIDDK under ARRA Challenge Grants RC1DK086542 and RC1DK086542.

