About Our Research
Current Research
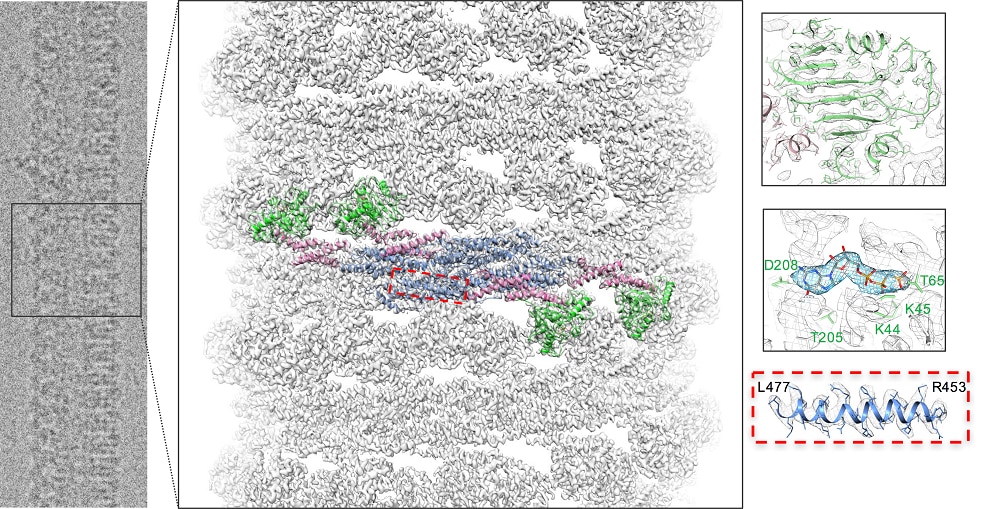
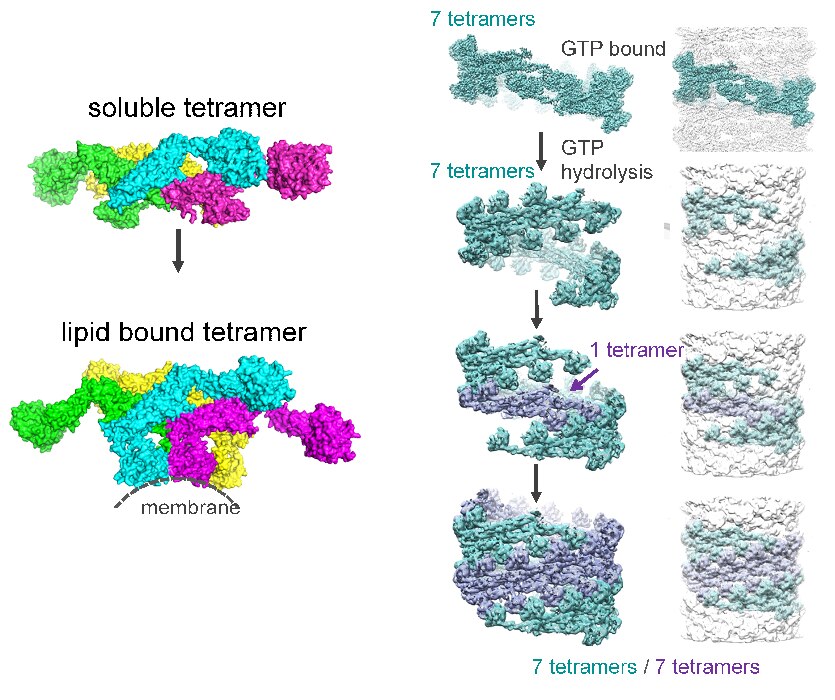
The dynamin family of proteins contains unique GTPases involved in membrane fission and fusion events throughout the cell. Our goal is to understand the dynamic structural properties of these proteins and correlate them with their diverse cellular functions. The founding member, dynamin, is crucial for endocytosis, synaptic membrane recycling, and membrane trafficking within the cell, and is associated with filamentous actin. Mutations in dynamin have been shown to cause the peripheral neuropathy, Charcot-Marie-Tooth disease (CMT), centronuclear myopathy (CNM) and hereditary spastic paraplegia (HSP). Over the years, structural studies have played a leading role in dissecting the function of dynamin in membrane fission. We have shown that purified dynamin readily assembles into rings and spirals and forms similar structures on liposomes, generating dynamin-lipid tubes that constrict and twist upon GTP hydrolysis. The ability of dynamin to constrict and generate a force on the underlying lipid bilayer makes it unique among GTPases as a mechanochemical enzyme. Our high-resolution cryo-EM structure of human dynamin 1 (3.7 Å) allowed us to build an atomic model of dynamin in the biologically active assembled state. In addition, cryo-EM structures of dynamin in different nucleotide states provided a model for dynamin-driven membrane fission.
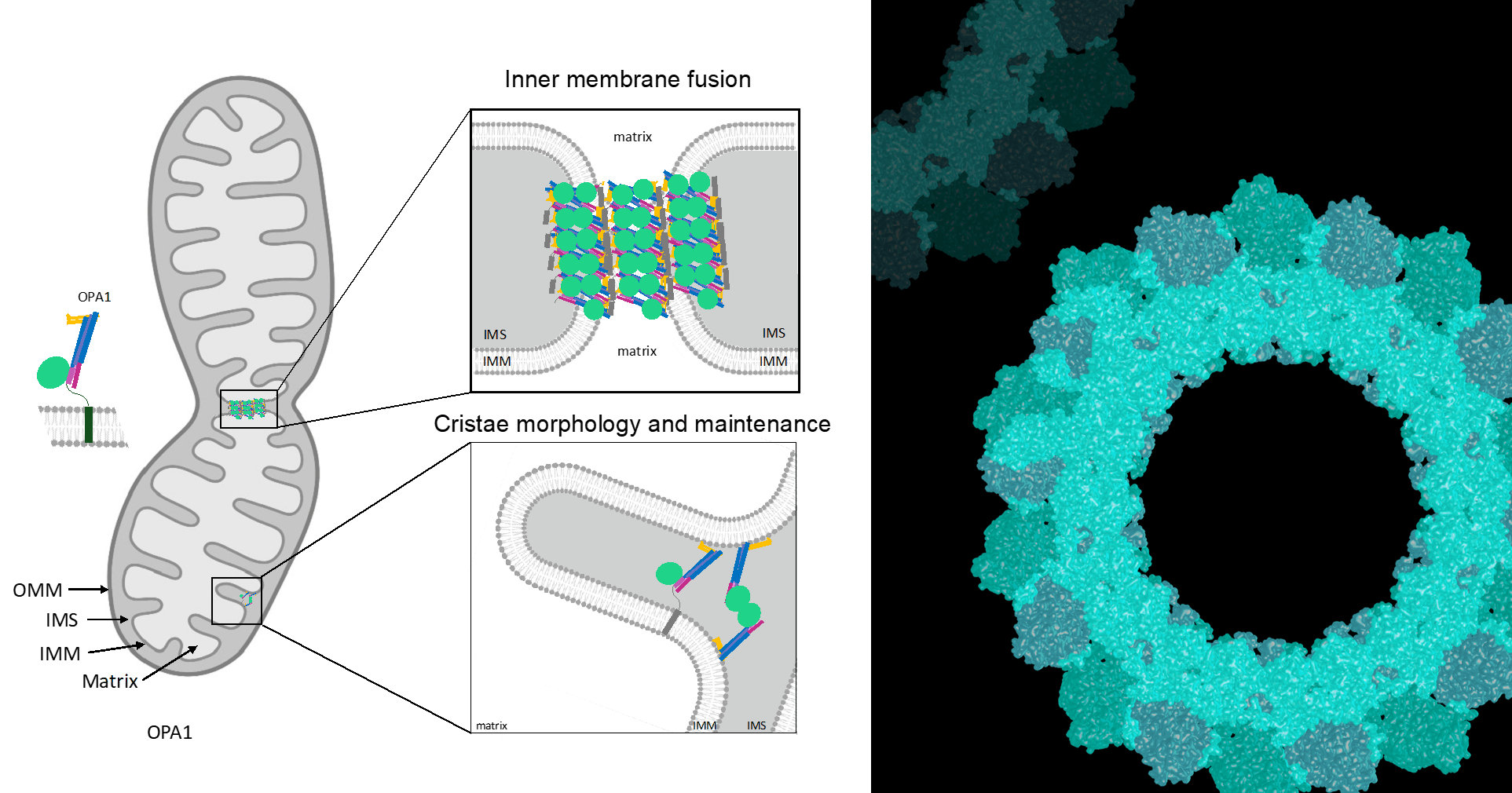
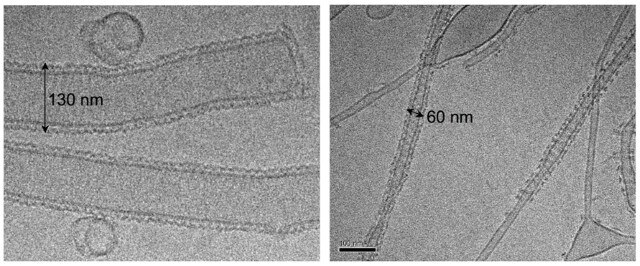
Additional dynamin family members have been implicated in a variety of fundamental cellular processes, including mitochondrial fission and fusion, antiviral activity, plant cell plate formation, and chloroplast biogenesis. Among these proteins, self-assembly and oligomerization into ordered structures is a common characteristic and, for the majority, is essential for their function. Although there is a wealth of information regarding dynamin, less is known about the structural properties of dynamin-related proteins. In the cell, healthy mitochondria are maintained by multiple cycles of fusion and fission. The dynamin-related protein (Drp1 in mammals or Dnm1 in yeast) is essential for mitochondrial fission and localizes to sites of mitochondrial division. We solved the structure of Dnm1-lipid tubes by cryo-electron microscopy and in comparison to dynamin, the structure of Dnm1 is significantly larger in diameter and has a minimal attachment to the underlying lipid bilayer. However, similar to dynamin, the Dnm1-lipid tubes constrict from ~120 nm to ~60 nm in diameter upon GTP hydrolysis, followed by a rapid dissociation from the lipid bilayer. These results imply that Dnm1 has the ability to impart a large contractile force at the site of membrane fission during mitochondrial division. The dynamin-related protein OPA1 is involved in the fusion of inner-mitochondrial membranes. Mutations within this protein are a major cause of dominant optic atrophy (DOA) due to the neuropathy of retinal ganglion cells. There exists a short and long form of OPA1 and both are necessary for mitochondrial fusion. We have characterized several mutants of OPA1 associated with DOA and showed that the short form self-assembles around liposomes forming protein-lipid tubes. The OPA1 mutants reveal defects in lipid binding, GTP hydrolysis, and membrane tubulation.
Applying our Research
Dynamin is necessary for internalizing essential nutrients, is tightly coupled to cell signaling events, and has been linked to the neuropathy CMT and the myopathy CNM. Mutations in the dynamin-related protein OPA1 result in autosomal dominant optic atrophy (inherited DOA). A mutation in the mitochondrial dynamin-related protein, Drp1, has also been linked to a server case of CMT and DOA. Mx dynamin-like proteins are interferon-induced with antiviral activity against a range of RNA viruses, including influenza viruses and members of the bunyavirus family. Examining the diverse dynamin family members will determine if a common mechanism governs this GTPase family and provide insight into dynamin-associated diseases. This knowledge may be used to help scientists develop treatment methods for these diseases and may possibly lead to prevention some day through gene therapy.
Need for Further Study
We are beginning to understand the mechanism of action for dynamin in membrane fission; however, how this protein is regulated in the cell is not clearly understood. In addition, it remains a mystery the mechanism of how dynamin family members are also involved in membrane fusion.
Research Images