Determining Drug Dosing in Adults with Chronic Kidney Disease
Knowing a patient’s kidney function when dosing medicine excreted by the kidneys is important to prescribing the correct amount of medicine for the patient.
Selecting Estimating Equations to Determine Kidney Function
The U.S. Food and Drug Administration (FDA) does not favor or recommend the use of a specific estimating equation for assessing drug dosage adjustments in patients with impaired kidney function. General recommendations are to adopt newer, more accurate equations as they evolve.
Despite the differences in estimating equations, there are often minimal differences in the drug dosage adjustments calculated, regardless of the equation used to estimate kidney function. A large simulation study in 2009 compared estimated glomerular filtration rate (eGFR) and estimated creatinine clearance (eCrCl) calculated from standardized creatinine values to each other and to gold-standard measurements of GFR. The results of the study suggest that, for the majority of patients and for most drugs tested, there was little difference in the recommended drug dose.1
Currently, the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) creatinine- and cystatin C-based equations, notably the 2021 CKD-EPI equations,2 are the newest and should be considered when possible. When the eGFR may have a direct effect on drug dosing, notably when the eGFR is close to a drug dosage cut off point, consider use of the combined 2021 CKD-EPI creatinine-cystatin C equation, which provides higher accuracy and decreased bias.
Considerations for Body Surface Area
Body surface area (BSA) is an important factor when estimating GFR because kidney function is proportional to kidney size, which is proportional to BSA. As such, BSA adjustment is necessary when comparing a patient's kidney function to normal values or to the levels defining the stages of CKD.
However, while adjusting for BSA may help assess if a patient’s kidney function is proportional to his or her body size, it may distort the association with drug clearance, as clearance of drugs is only related to GFR and not to body size or BSA. Therefore, adjusting for BSA is not usually necessary for determining drug dosing except in patients whose body size is very different than average.
Some of the most commonly used eGFR equations—such as the CKD-EPI and Modification of Diet in Renal Disease (MDRD) Study equations—estimate GFR adjusted for BSA. Given that the estimating equations that adjust for BSA assume an average BSA of 1.73m2, you can remove the correction for BSA in patients with BSA different than the standard (1.73m2) by multiplying the reported eGFR by the estimated BSA and divide by 1.73m2.
The eGFR (mL/min) for drug dosing can be expressed as: eGFR (mL/min/1.73 m2) x BSA/1.73
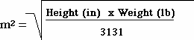
Note: BSA can be obtained from a standard nomogram or can be calculated using equations such as3:
Limitations of Serum Creatinine-based Estimating Equations
Serum creatinine-based estimating equations have limitations that should be considered when assessing kidney function and drug dosing. The serum concentration of creatinine is influenced by factors other than GFR—differences in size, muscle mass, and diet—as well as differences in the rate of tubular secretion. Measurement of GFR using cystatin C, measured creatinine clearance (CrCl), or exogenous filtration markers should be considered for people in whom estimates based on serum creatinine alone may be inaccurate, such as patients with very large or small body sizes, or when prescribing drugs with narrow therapeutic indices.4
Dosage Adjustments on FDA Drug Labels
FDA-approved drug labels provide information to inform drug dosage for patients with impaired kidney function. Dosing recommendations on drug labels have been developed using a variety of methods to assess kidney function, including
- serum creatinine
- measured CrCl
- eCrCl using the Cockcroft-Gault, MDRD Study,5 or CKD-EPI (20096 and 2021) equations
Drugs developed before 2005 may have labels developed prior to the standardized calibration of creatinine assays and reporting eGFR calculated using the MDRD Study and CKD-EPI equations. Given the evolution of eGFR methods and timing of when drugs were approved, the dosing recommendation for some drugs may be based on estimating equations that are no longer the most accurate estimates. Read about the FDA’s guidance on pharmacokinetics and drug dosing in individuals with impaired renal function.
References
This content is provided as a service of the National Institute of Diabetes and Digestive and Kidney Diseases
(NIDDK), part of the National Institutes of Health. NIDDK translates and disseminates research findings to increase knowledge and understanding about health and disease among patients, health professionals, and the public. Content produced by NIDDK is carefully reviewed by NIDDK scientists and other experts.

